In active talks on partnership with a Japanese partner; overseas expansion likely to be introduced soon
AIDOT (CEO Jeong Jae-hoon) announced that its AI-based gastroscopy interpretation system has received clinical trial plan approval from the Ministry of Food and Drug Safety (MFDS) under the category of “gastric cancer image detection diagnostic support software.”
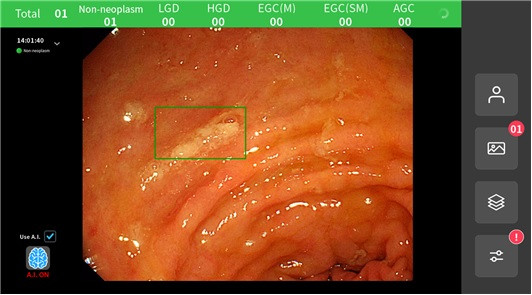
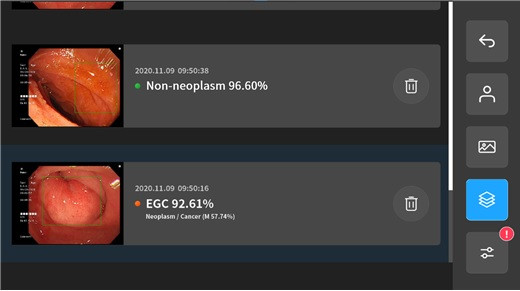
AIDOT’s AI-based gastroscopy solution was developed together with a team led by Professor Bang Chang-seok of the Division of Gastroenterology at Hallym University Chuncheon Sacred Heart Hospital. The solution classifies gastroscopy images into six severity categories: △Non-neoplasm △LGD △HGD △EGC (M) △EGC (SM) △AGC. According to the company’s internal clinical evaluation results, the overall sensitivity and specificity—reflecting sensitivity and specificity by severity—were 91.48% and 92.13%, respectively.
AIDOT also said that during gastroscopy procedures, the system immediately marks the location of suspected lesions based on real-time video, making it an optimal diagnostic support system that can assist physicians directly at the point of care.
An AIDOT official said, “Existing gastroscopy equipment is dominated by devices from specific countries and companies, accounting for 80% of the global market share,” adding, “In this quasi-monopolistic environment, AIDOT is presenting an integrated package AI solution that combines hardware and software to simplify and optimize compatibility across various image formats and ports used by existing gastroscopy equipment. We are currently in active discussions with a Japanese partner—Japan is often called the ‘home country of gastroscopy equipment’—regarding solution purchases and a strategic partnership.”
Im Yoon-jae, head of AIDOT’s Business Planning Division overseeing regulatory approvals, said, “This MFDS approval of the clinical trial plan served as an opportunity to recognize the globalization of an AI gastroscopy solution that has not existed in the world market to date,” adding, “We plan to complete regulatory approval by achieving excellent results through this clinical trial. We also plan to complete approval procedures in the near future for our already developed colonoscopy and capsule endoscopy solutions and introduce an integrated solution for the gastroenterology field.”
About AIDOT
Since its founding in June 2014, AIDOT has been recognized for its technological capabilities, including being selected for KIC China and various government projects. It developed the AI-based cervical cancer interpretation system “Cerviray A.I.” and is expanding into global markets centered on Southeast Asia. It also co-developed an AI-based interpretation system combining carotid ultrasound and genomic information with the Department of Neurosurgery at Hallym University Chuncheon Sacred Heart Hospital, and it has completed development of an AI diagnostic system for gastroenterology integrating gastroscopy, capsule endoscopy, and colonoscopy.
Website: aidot.ai
Published: September 7, 2022 / Newswire