AIDOT, an AI-based medical ICT company, announced on the 2nd that its AI-based cervical cancer interpretation system, “Cerviray AI,” has obtained certification of compliance with Good Manufacturing Practice (GMP) standards for medical device manufacturing and quality management. The product is classified as a Class III medical device under the category of physiological monitoring devices.
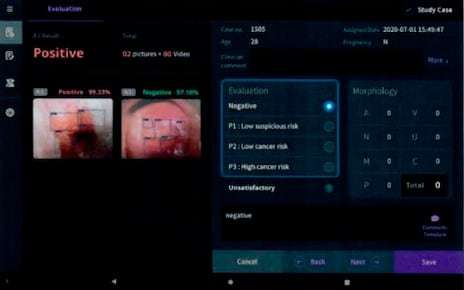
GMP (Good Manufacturing Practice) is a quality assurance system managed by the Ministry of Food and Drug Safety (MFDS) to verify the safety and effectiveness of medical devices manufactured by medical device companies and to ensure that they are produced consistently at a level of quality appropriate for their intended use. Cerviray AI was certified as “medical image-assisted diagnostic software.”
Medical image-assisted diagnostic software automatically displays the likelihood and degree of factors such as △ the presence of disease, △ severity, and △ clinical condition based on medical images, thereby supporting healthcare professionals in making diagnostic decisions.
AIDOT plans to pursue Class III certification from the MFDS for its other completed solutions, including an AI-based stroke interpretation system, also under the category of AI-based medical image-assisted diagnostic software.
For overseas commercialization, AIDOT has completed regulatory approval procedures with its global partners, including CE certification in Europe and approval from the China Food and Drug Administration (CFDA). Through these certifications, the company has achieved active export performance in China, Europe, and Southeast Asia.
AIDOT stated that it has completed all necessary preparations, including domestic clinical trial procedures, to re-enter the Korean market in early 2021, and that it is currently engaged in in-depth preliminary discussions with multiple domestic partner companies to obtain MFDS approval.
Kim Deok-yeol, Head of Development, said, “We have established a business model that enables a win-win partnership with domestic medical professionals,” adding, “We hope that our diagnostic support software can provide significant benefits to doctors, patients, and companies alike, not only in the domestic cervical cancer diagnosis market but also in the stroke diagnosis market.”
Chosun Biz / Reporter Jang Yoon-seon
Published: September 2, 2020, 10:08