Key solution: the FDA’s 510(k) premarket clearance
Latin America is cited as a top strategic export region.
Korean venture companies are being urged to think carefully about market entry strategies.
Apple’s wearable device, the Apple Watch, includes an atrial fibrillation function that can check heart rhythm. That technology is listed under the U.S. Food and Drug Administration (FDA) medical-device 510(k) premarket clearance category. Most products in the AI medical market have received approval under this category, and the market size has grown about fourfold in three years to roughly KRW 20 trillion, prompting calls for Korean companies to more actively consider entering the market.
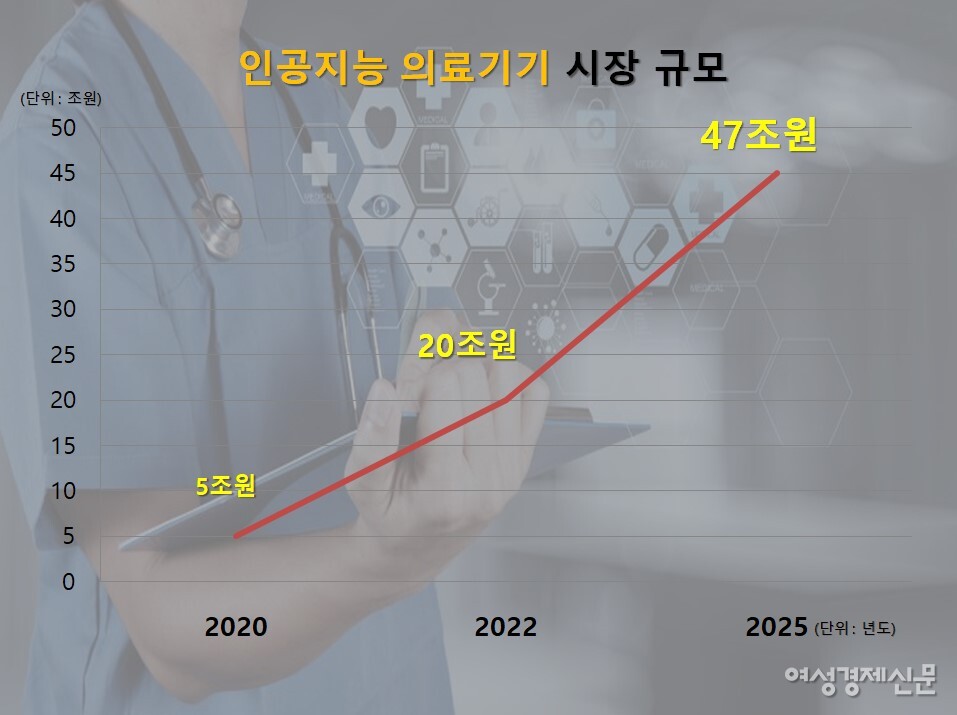
On the 15th, citing market research firms and other sources, the article said the global AI healthcare market grew rapidly from KRW 5.8 trillion (USD 4.5 billion) in 2020 to about KRW 20.3 trillion (USD 15.6 billion). By 2025, the market is expected to expand to KRW 47 trillion.
About 47% of the AI healthcare market is the U.S. market, where global big tech companies such as Apple and Alphabet (Google’s parent company) are described as holding a near semi-monopolistic position. According to data disclosed by the FDA, 91 medical devices related to AI and machine learning (ML) health solutions received FDA clearance from January to October of last year.
The article adds that, for AI medical devices, Latin America excluding Brazil is often considered the largest export market. A representative from a domestic medical-device company told the outlet that while most countries—including Europe, Japan, Korea, and the United States—have their own medical-device certification systems, many Latin American countries can substitute FDA approvals as reference documents for import certification.
In other words, if a company’s AI medical device receives FDA approval, it may be able to secure a so-called “1+1” market—covering not only the United States but also Latin America. Korea is also said to have the advantage of being able to enter Latin American markets by cooperating with local health authorities through KOICA (Korea International Cooperation Agency) support programs.
To clear the export barrier in the United States and Latin America, the article states that an AI medical device must obtain the 510(k) category. An official from Korea’s Ministry of Food and Drug Safety (MFDS) said this category signifies that a manufacturer has demonstrated safety for distribution by evaluating the device’s intended use, technical characteristics, and performance testing, and that it can be marketed in the United States or in countries that apply FDA standards.
In Korea, the list of companies that have received FDA product clearance is narrowed to Lunit, Huron, Coreline Soft, ClariPiA, iMedicine, and AIDOT, which have developed AI medical devices related to breast cancer, Parkinson’s disease, and cervical cancer.
The industry views FDA approval for Korean AI medical-device ventures as meaning they can also anticipate better prospects for market entry and competitiveness in Latin America. Jeong Jae-hoon, CEO of AIDOT, said in this regard, “In our case, we contacted the Bolivian Ministry of Health through a KOICA program,” adding, “The Ministry of Health is welcoming our exports, so I think we may achieve concrete results within the first half of this year.”
Another medical-device industry official added that obtaining or being listed with the U.S. FDA can make entry into Latin American markets such as Brazil and Argentina relatively easier, and said that, for Korean companies, the AI medical-device market is “prime territory” that must be opened up.
Published: March 15, 2023 / Woman Economy / Reporter Kim Hyun-woo