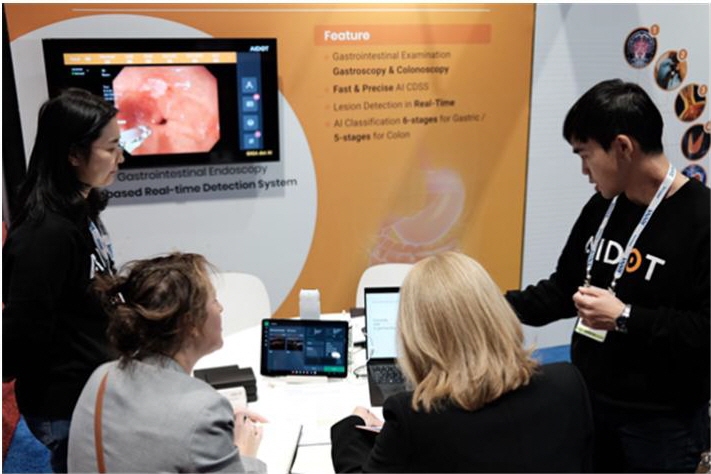
AIDOT (CEO Jung Jae-hoon) announced that at RSNA 2023 (the Radiological Society of North America 2023), held in Chicago, USA from November 26 to 29, it arranged pre-scheduled meetings with leading global medical device companies and conducted in-person discussions.
Through this, the company said that GIGA dot AI, an AI gastroscopy solution, and SONO dot AI, an AI solution for carotid ultrasound, both of which had registered MFDS Class 3 approvals this year, received strong interest, and that it agreed to continue additional joint research with global companies.
In particular, SONO dot AI has a function that uses AI to assess in advance the risk that plaque in the carotid artery area may break off and block cerebral blood vessels, leading to cerebral infarction (ischemic stroke), and to notify users of that risk.
Regarding this function, the company explained that it received strong interest from North American and European ultrasound device companies, and that it received “love calls” for licensing adoption because, through a guide function that automatically recognizes the location of the carotid vessel using AI and a detection function that detects the location of plaque, it can identify the carotid artery and plaque more accurately and easily.
Lim Ga-ram, Head of Global Marketing & Sales at AIDOT, who oversaw participation in this exhibition, said, “Before participating in the exhibition, conducting sufficient online meetings in advance had a big effect,” and added, “Through this in-person meeting, we agreed on additional joint research to enhance racial differences by carotid region, and at the same time, it is very meaningful that we can develop it into a globally customized solution suited to the North American and European markets by distributing the solution in a licensing format.”
An AIDOT official said, “SONO dot AI has completed approval of a Class 2 AI medical device clinical trial plan from the Korean Ministry of Food and Drug Safety (MFDS), and we will continue clinical trials that can be applied not only domestically but also in global markets.”
Korea Economic TV / 2023.11.30 / Senior Reporter Yang Jae-joon